With a tendency to impair endolysosomal and lysosomal function and subsequently exhibit neurotoxicity, the mutation of protein leucine-rich repeat kinase 2 (LRRK2) has grown into one of the leading causes for Parkinson’s disease (PD) [1][2]. In our quest to continuously improve drug efficacy, Nextcea has identified and linked Parkinson’s from the LRRK2 mutation with our patented biomarker di-22:6-BMP.
di-22:6-BMP Biomarker
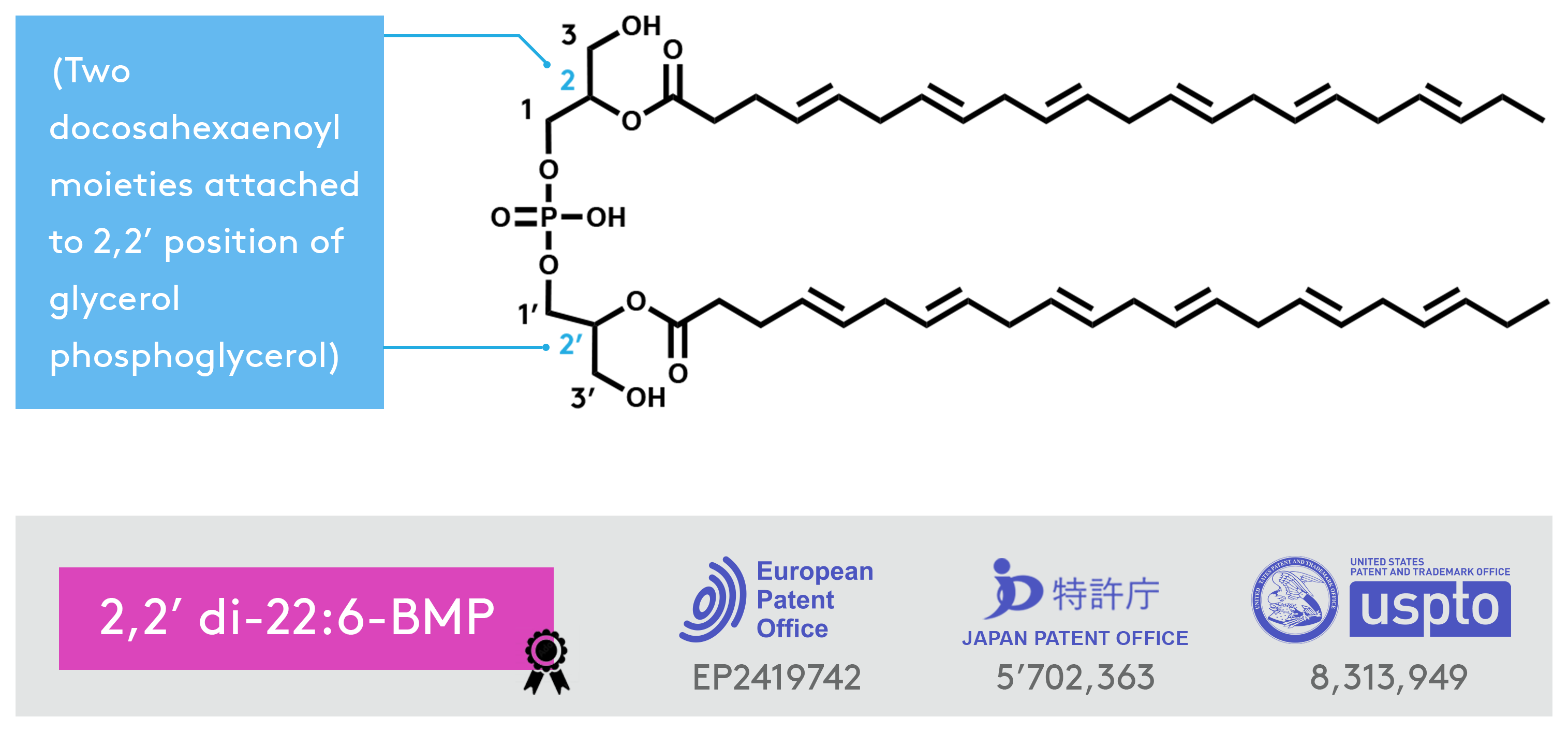
Di-docosahexaenoyl (22:6)-BMP
Bis(monoacylglycerol)phosphate (BMP) is a unique lysosomal phospholipid that plays important roles in lysosomal degradation pathways. The di-22:6-BMP, a specific species of BMP, is Nextcea’s patented biomarker of lysosomal storage dysfunction for Parkinson’s disease (LRRK2 mutation) drug efficacy assessments(/therapeutic uses) and diagnostics. The di-22:6-BMP is measured in urine to evaluate the impact of LRRK2 kinase inhibitors on lysosomal functions.
In collaboration with the Michael J Fox Foundation among additional academic institutions, Nextcea has confirmed a correlation between Parkinson’s patients from the LRRK2 G2019S, R1441G mutations and elevated levels of di-22:6-BMP biomarker in urine. Contrary to DNA mutation assays’ sole ability of only identifying Parkinson’s, di-22:6 BMP as a biomarker “opens [additional] avenues for future studies to establish the utility of urinary BMP as a biomarker to monitor disease progression and as a pharmacodynamic biomarker to demonstrate LRRK2 kinase modulation by drug candidates” (MovementDisorders,Vol. 35, No. 1, 2020)―most significantly, efficacy assessment of LRRK2 drug candidates and patient selection and stratification during clinical studies.
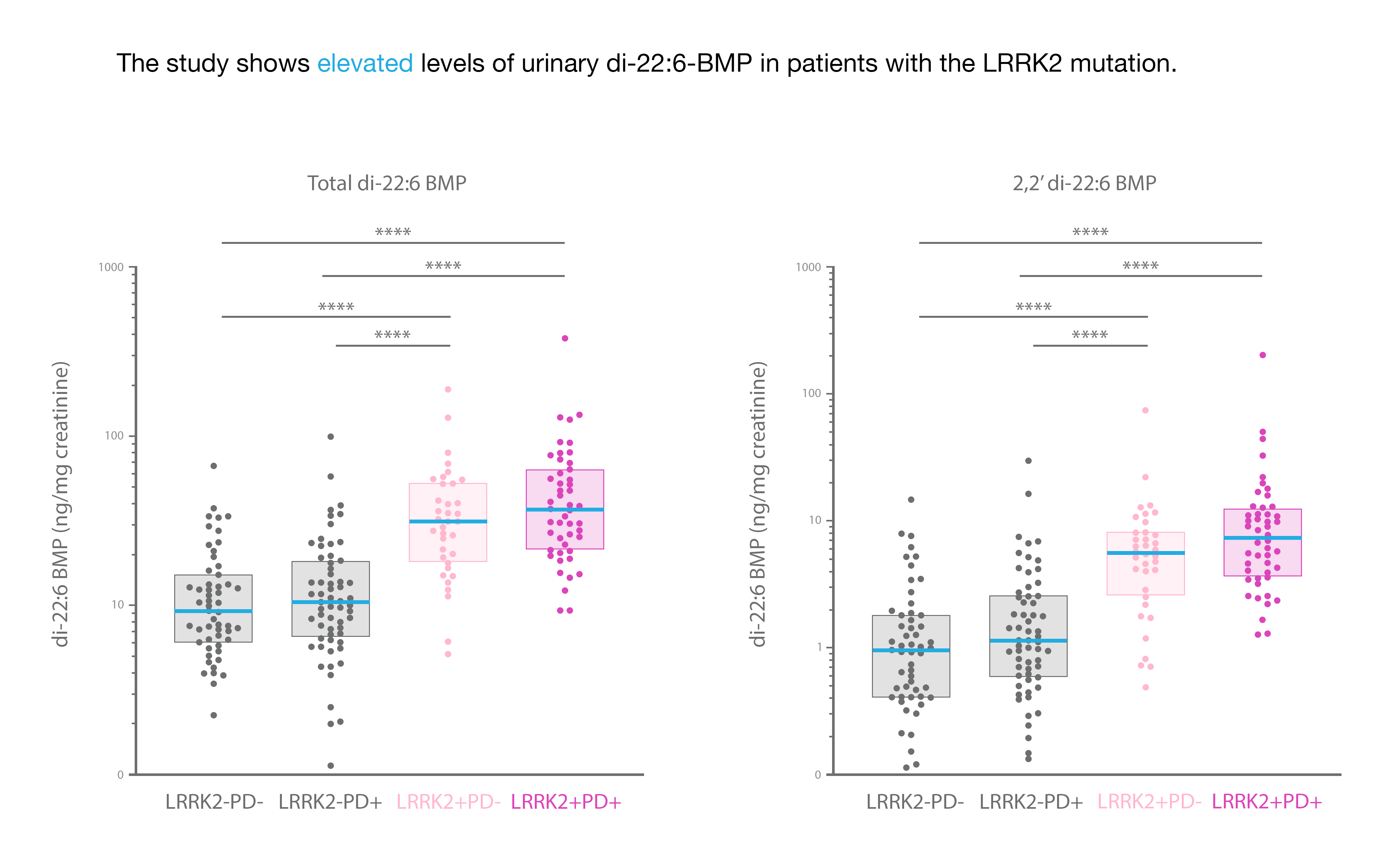
Proprietary biomarker di-22:6-BMP in urine analyzed at Nextcea as a validated biomarker for the LRRK2 mutation
In Figure 1, urine was collected from healthy controls (LRRK2-PD-), sporadic PD patients (LRRK2-PD+), non-manifesting carriers i.e. without PD (LRRK2+PD-), and LRRK2 mutation carriers (LRRK2+PD+). Di-22:6-BMP and its 2,2’-di 22:6-BMP active isomer were quantitated, with an authentic reference standard and internal isotopic standard, by UPLC-MS/MS. Biomarker concentrations in urine were normalized to urine creatinine.
Nextcea offers quantitation of di-22:6 BMP in order to assess the efficacy, dosing regimen, & dosing schedule of LRRK2 inhibitors
Determining efficacy of LRRK2 inhibitors
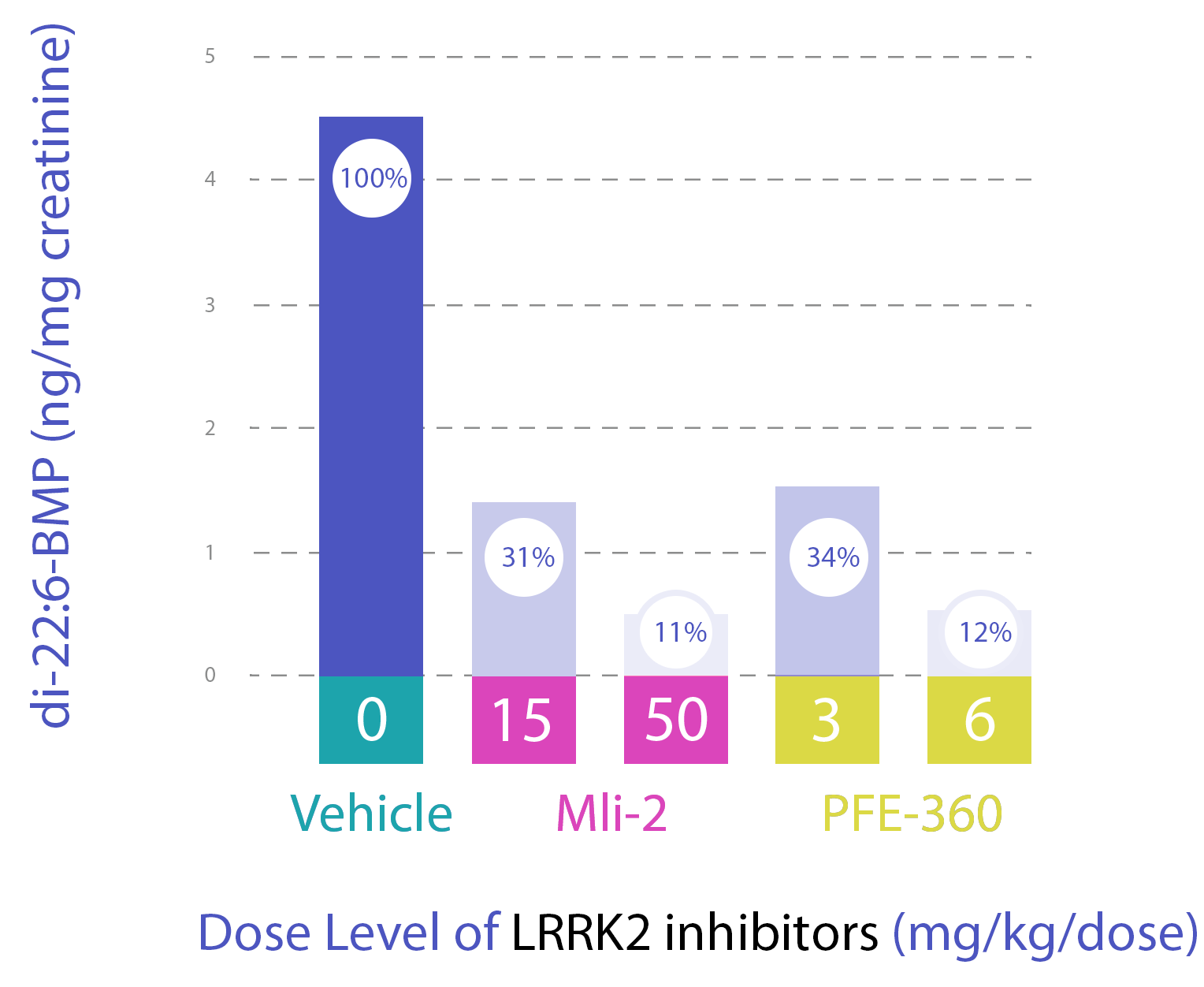
In Figure 2A, urine di-22:6-BMP decreased following administration of LRRK2 inhibitors (MLi 2, PFE 360, and GNE-7915) in nonclinical animal studies presented by Michael J Fox Foundation [1][2].
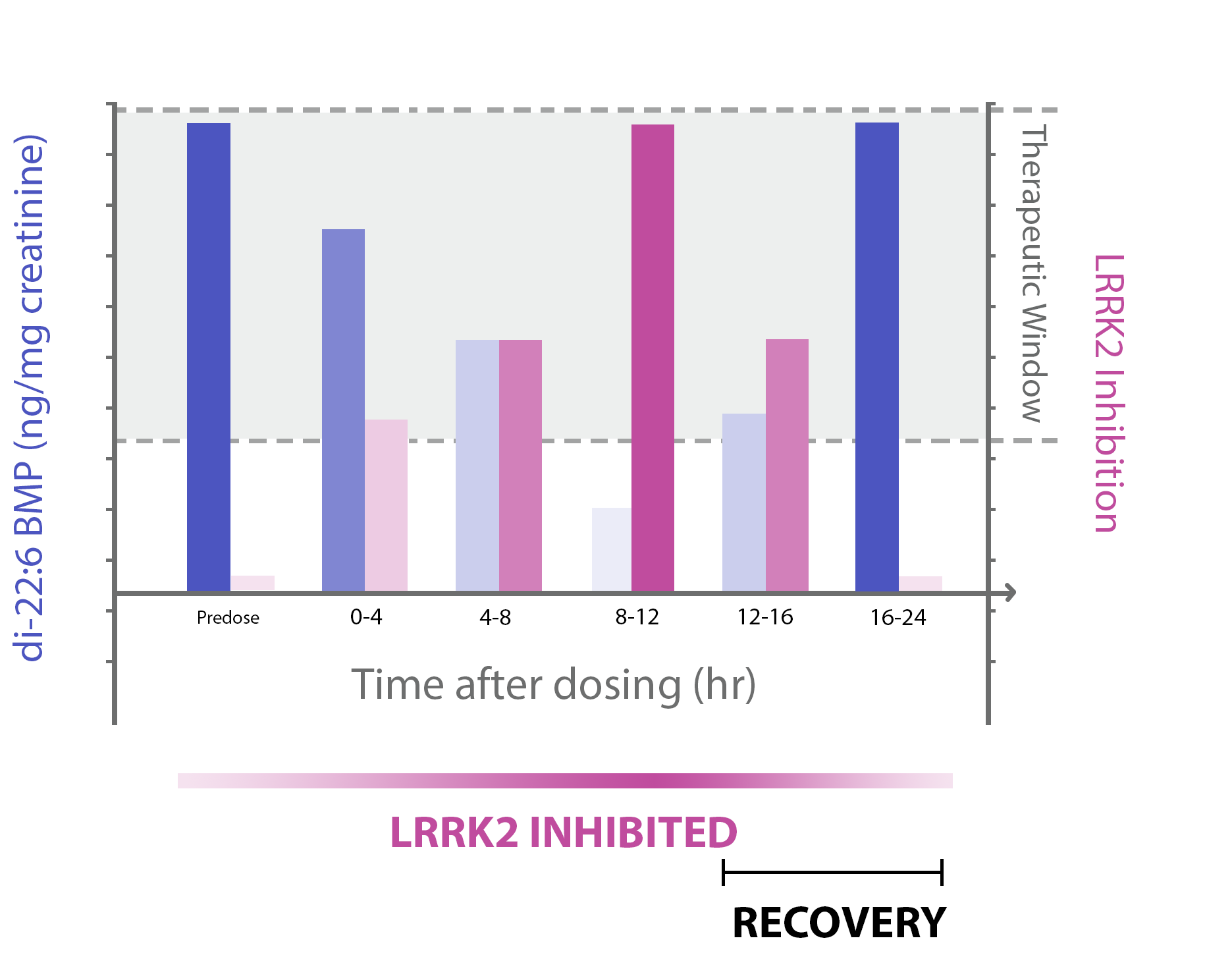
Determining Therapeutic Window, Dosing Regimen & Dosing Schedule for LRRK2 inhibition drug candidates by pharmacodynamic di-22:6 BMP biomarker
As illustrated in Figure 2B, urine di-22:6-BMP decreased following administration of LRRK2 inhibitors but was recovered back to predose levels (and waned in LRRK2 inhibition) after an observed amount of prolonged time. By determining when recovery incurs, dosing regimen and dosing schedule can be calculated for LRRK2 drug candidates. (The grey background represents the therapeutic window–an area of space where the LRRK2 drug candidate can be deemed “effective”. LRRK2 Inhibition not reaching the therapeutic window is classified as ”not effective”–i.e. “Predose” and hrs “16-24”. )
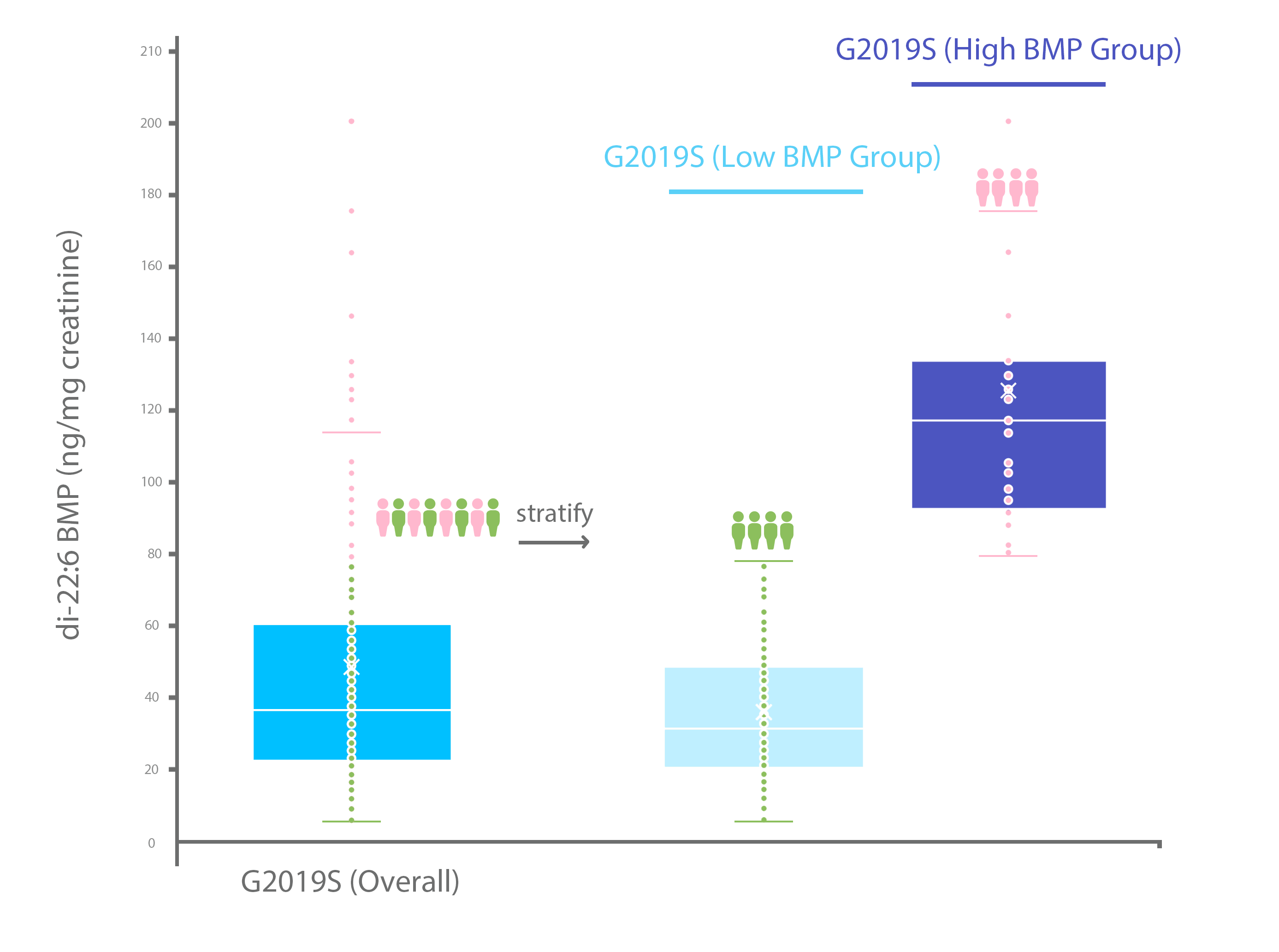
Quantitating di-22:6 BMP in GLP clinical trial studies at Nextcea in order to determine patient selection and stratification for LRRK2 drug candidates
As seen in Figure 3, Nextcea is capable of using measurements of di-22:6 BMP in urine to identify and categorize LRRK2 patients according to levels of di-22:6 BMP (i.e. a high BMP group and a low BMP group). By narrowing the appropriate di-22:6 BMP range to select patients from, clincial studies can realize considerable more success due to a predetermined effectiveness the LRRK2 drug candidates may already have with patients of a certain di-22:6 BMP range.
References
- Merchant et al (2023) LRRK2 and GBA1 variant carriers have higher urinary bis(monacylglycerol) phosphate concentrations in PPMI cohorts 9:30; https://doi.org/10.1038/s41531-023-00468-2
- Gomes et al (2023) Elevated urine BMP phospholipids in LRRK2 and VPS35 mutation carriers with and without Parkinson’s disease. Npj Parkinsons disease 9:52; https://doi.org/10.1038/s41531-023-00482-4
- Meneses-Salas et al (2023) Exosome-mediated release of bis(monoacylglycerol)phosphate is regulated by LRRK2 and Glucocerebrosidase activity bioRxiv https://doi.org/10.1101/2023.07.12.548710
- Fujjii RN,, Fllagelllla M,, Baca M,, Bapttiistta MAS ett all.. ((2015)) Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Science Translational Medicine. 7(273): 273ra15. doi: 10.1126/scitranslmed.aaa3634
- Baptista MAS, Merchant K, Bryce D, Ellis M, et al. (2015) LRRK2 kinase inhibitors of different structural classes induce abnormal accumulation of lamellar bodies in type II pneumocytes in non-human primates but are reversible an without pulmonary functional consequences.
https://www.michaeljfox.org/publication/lrrk2-kinase-inhibitors-different-structural-classes-induce-abnormal-accumulation - Miranda AM, Lasiecka ZM, Xu Y, Neufeld J, Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA, Di Paolo G (2018). Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat Commun 9(1):291. doi: 10.1038/s41467-017-02533-w
- Miranda AM, Di Paolo GD. (2018) Endolysosomal dysfunction and exosome secretion: implications for neurodegenerative disorders. Cell Stress. 2(5):115-118. doi: 10.15698/cst2018.05.136
- Alcalay RN, Hsieh F, Tengstrand E, et al (2020) Higher Urine bis(Monoacylglycerol)Phosphate Levels in LRRK2 G2019S Mutation Carriers: Implications for Therapeutic Development. Movement Disorders, 35(1):134-141
 Parkinson’s LRRK2 disease BMP biomarker
Parkinson’s LRRK2 disease BMP biomarker

 is here to assess drug efficacy better.
is here to assess drug efficacy better.