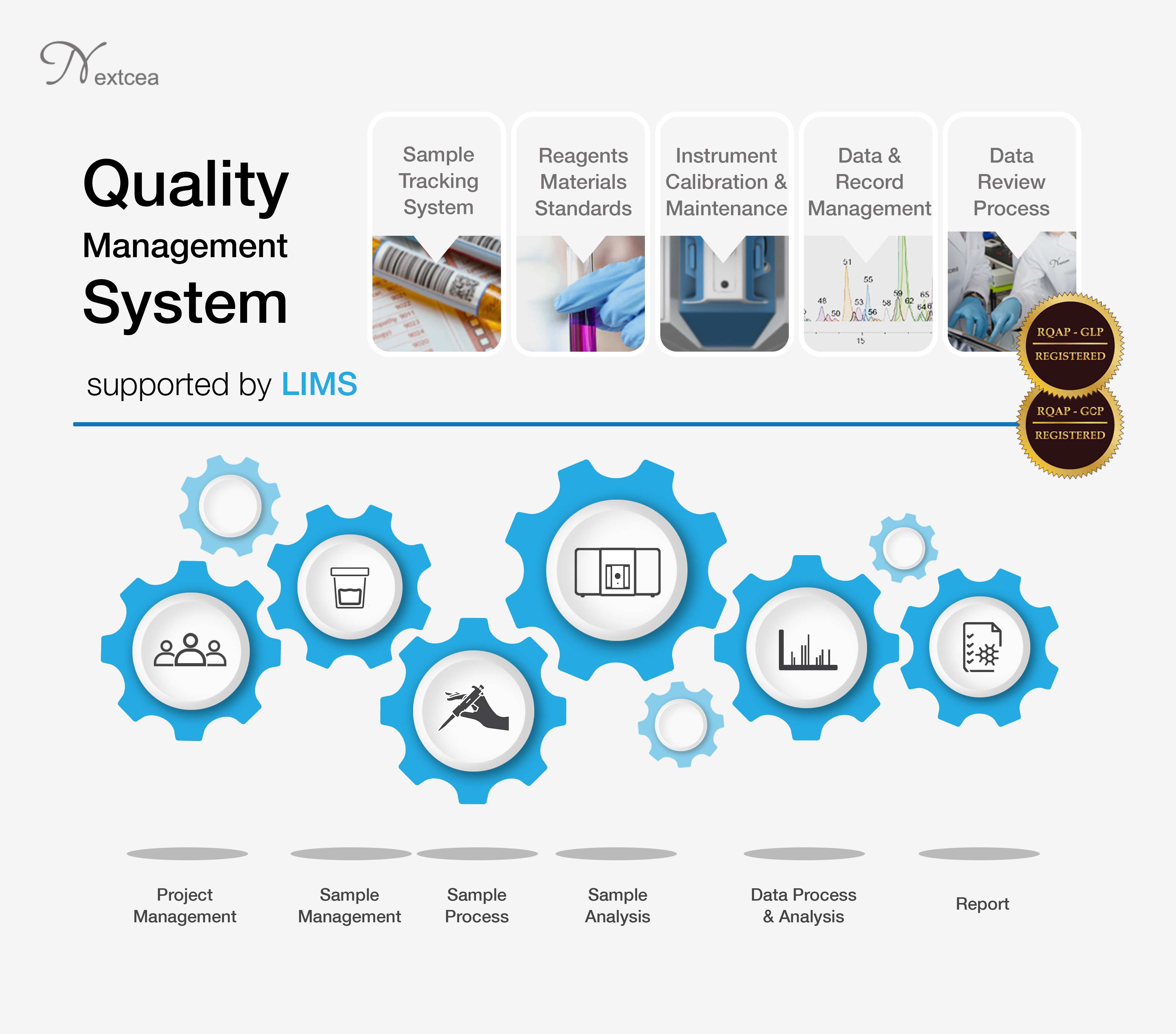
The Quality Assurance Unit (QAU) at Nextcea Inc. ensures data quality and study integrity in accordance with GLP regulations. Nextcea QAU staff members are Registered Quality Assurance Professionals in GLP. Each QAU staff member:

The Quality Assurance Unit (QAU) at Nextcea Inc. ensures data quality and study integrity in accordance with GLP regulations. Nextcea QAU staff members are Registered Quality Assurance Professionals in GLP. Each QAU staff member:
